当前位置:网站首页>PCI Pharma Services Announces Multi-Million Dollar Expansion of UK Manufacturing Facility to Meet Growing Demand for Global High Potency Drug Manufacturing Services to Support Oncology Treatment
PCI Pharma Services Announces Multi-Million Dollar Expansion of UK Manufacturing Facility to Meet Growing Demand for Global High Potency Drug Manufacturing Services to Support Oncology Treatment
2022-08-05 06:57:00 【sinat_41698914】
New facility provides innovative equipment and increased capacity to bring high-potency oncology therapies to market
The world's leading Contract Development and Manufacturing Organization (CDMO) PCI Pharma Services (PCI) today announced that the company has announced a new commitment to a world-class facility in Tredegar, Wales, UK.Major expansion of the facility to keep pace with market growth for potent targeted oncology therapies.
Salim Haffar, CEO of PCI Pharma Services, said: "We are delighted to announce our latest expansion of the Tready Processing facility. This will meet the urgent and growing need for global specialty manufacturing services in oncology.The ever-expanding demand for potent therapeutics and highly complex concentrated formulations can present unique challenges to manufacturing and packaging. We are proud to manage this area of expertise at clinical and significant commercial scale and be one of the few with this global capabilityone of the suppliers."
The expansion includes two new facilities dedicated to the manufacture and packaging of solid oral tablets and capsules.Building on the success of the original Closed Manufacturing Facility (CMF1), which opened in 2013, a second Closed Manufacturing Facility (CMF2) will double large-scale processing capacity, including commercial-scale dispensing of high-potency solid-dose products andFluidized bed granulation capability.In addition, PCI will build a new high-capacity, multi-product packaging facility with primary and secondary blister packs and bottling kits.Primary and secondary packaging lines provide a fully integrated process that provides end-to-end service to a global customer base.
In 2021, more than 1,300 speciesDrugs and vaccines in clinical trials are indicated for cancer, while two yearsThe former number was 1,100.Cancer therapies accounted for 25 percent of all drugs approved by the U.S. Food and Drug Administration between 2010 and 2019.This suggests that oncology will remain the focus of the industry for years to come.
Rebecca Coutts, Ph.D., general manager of PCI Pharma Services' Tready Processing Plant, said: "The rapid growth of the oncology pipeline coincides with the continued globalization of clinical development. This latest investment, along with existing analytical and formulation capabilities,The combination of clinical-scale and commercial-scale packaging facilities will complement existing clinical-scale manufacturing capabilities and further enhance commercial-scale manufacturing capabilities. The end-to-end manufacturing services for these high-potency molecules come together to better serve our customerschanging needs.”
Both facilities will contain state-of-the-art equipment, a large granulation facility replicating CMF1 and a laminar flow hood with a protective jacket.Building on CMF1's original state-of-the-art facility will provide customers with greater capacity and business continuity.
The expansion project in Trediga is expected to create up to 40 new jobs in its first year, with more jobs added as the facility expands.The PCI Treading facility currently employs nearly 500 people.Staff numbers have nearly doubled since CMF1 opened in 2013.PCI has been operating in Trediga, UK for almost 40 years and is one of the foremost employers there.To learn more about PCI's high-efficiency development and manufacturing capabilities, click here.
About PCI Pharma Services
PCI is a leading global contract development and manufacturing organization dedicated to providing customers with integrated end-to-end drug development, manufacturing and packaging capabilities to help customers accelerate time-to-market and improve their chances of commercial success.PCI has been in the healthcare services business for over 50 years and has a proven track record of successfully launching more than 50 products each year.We currently have 30 locations and more than 4,300 employees in seven countries (Australia, Canada, United States, Ireland, Wales, Germany and Spain) dedicated to bringing life-changing therapies to patients.Leading-edge technology and ongoing investment allow us to meet the needs of global drug development across the entire product lifecycle—from manufacturing capabilities, to the clinical trial supply chain, to commercialization.Our clients see us as an extension of their business and a partner in a shared commitment to improving patient lives.
边栏推荐
猜你喜欢
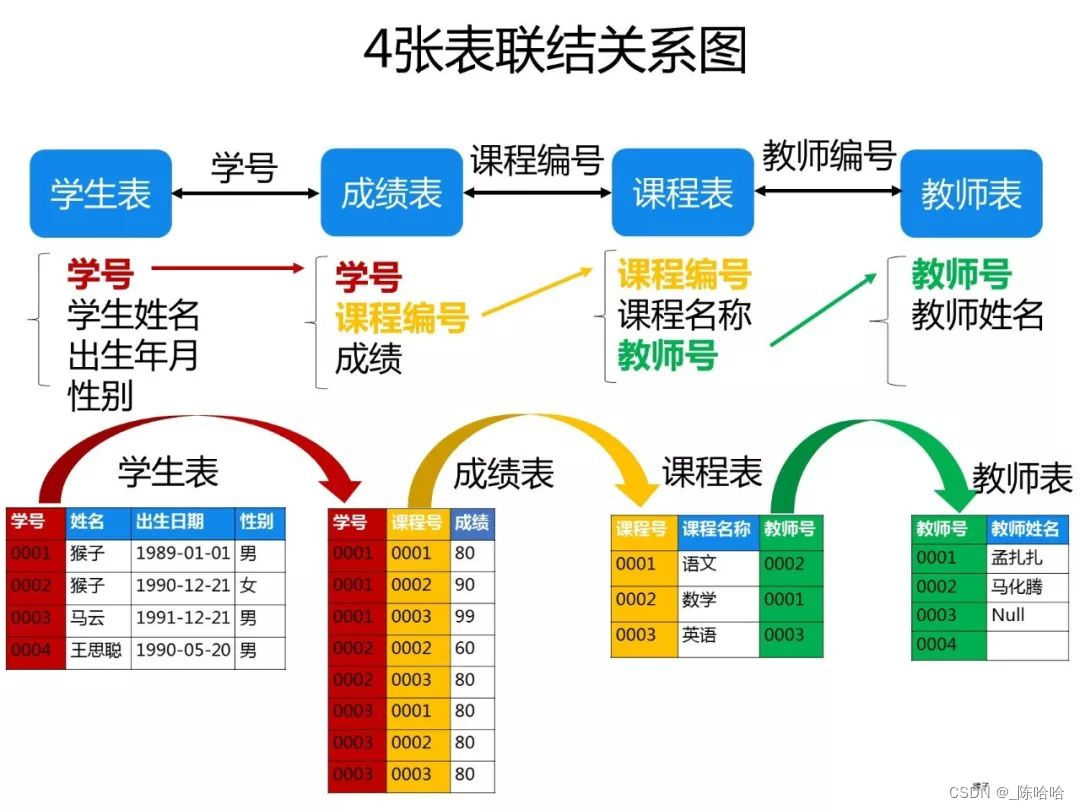
Late night drinking, 50 classic SQL questions, really fragrant~

【FAQ】CCAPI Compatible EOS Camera List (Updated in August 2022)

HelloWorld
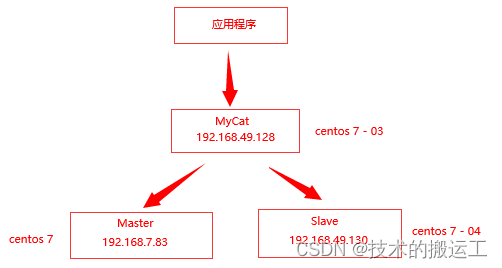
MyCat配置文件
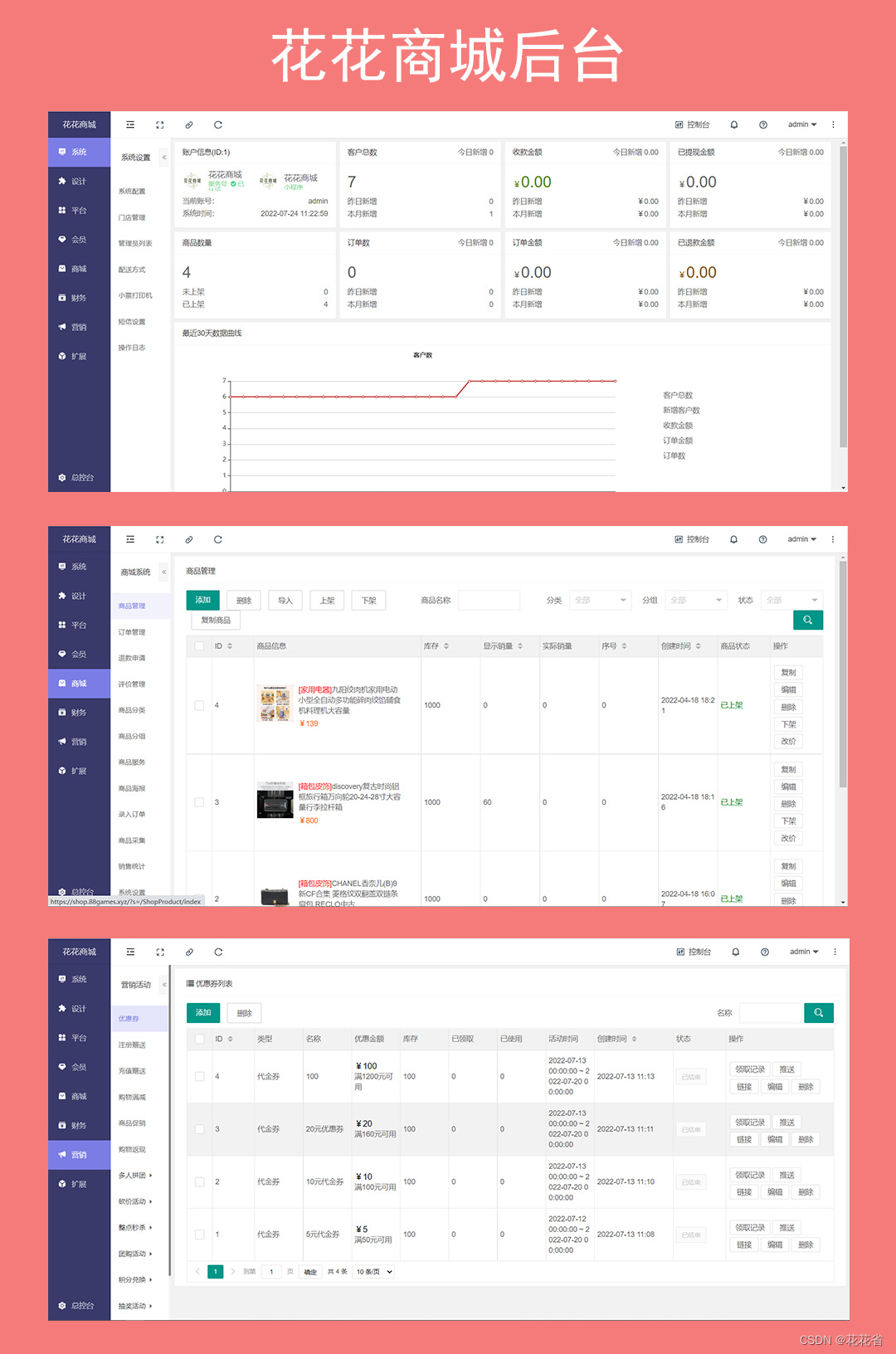
多用户商城多商户B2B2C拼团砍价秒杀支持小程序H5+APP全开源

Japan Sanitary Equipment Industry Association: Japan's warm water shower toilet seat shipments reached 100 million sets
Get the network input dimensions of the pretrained model
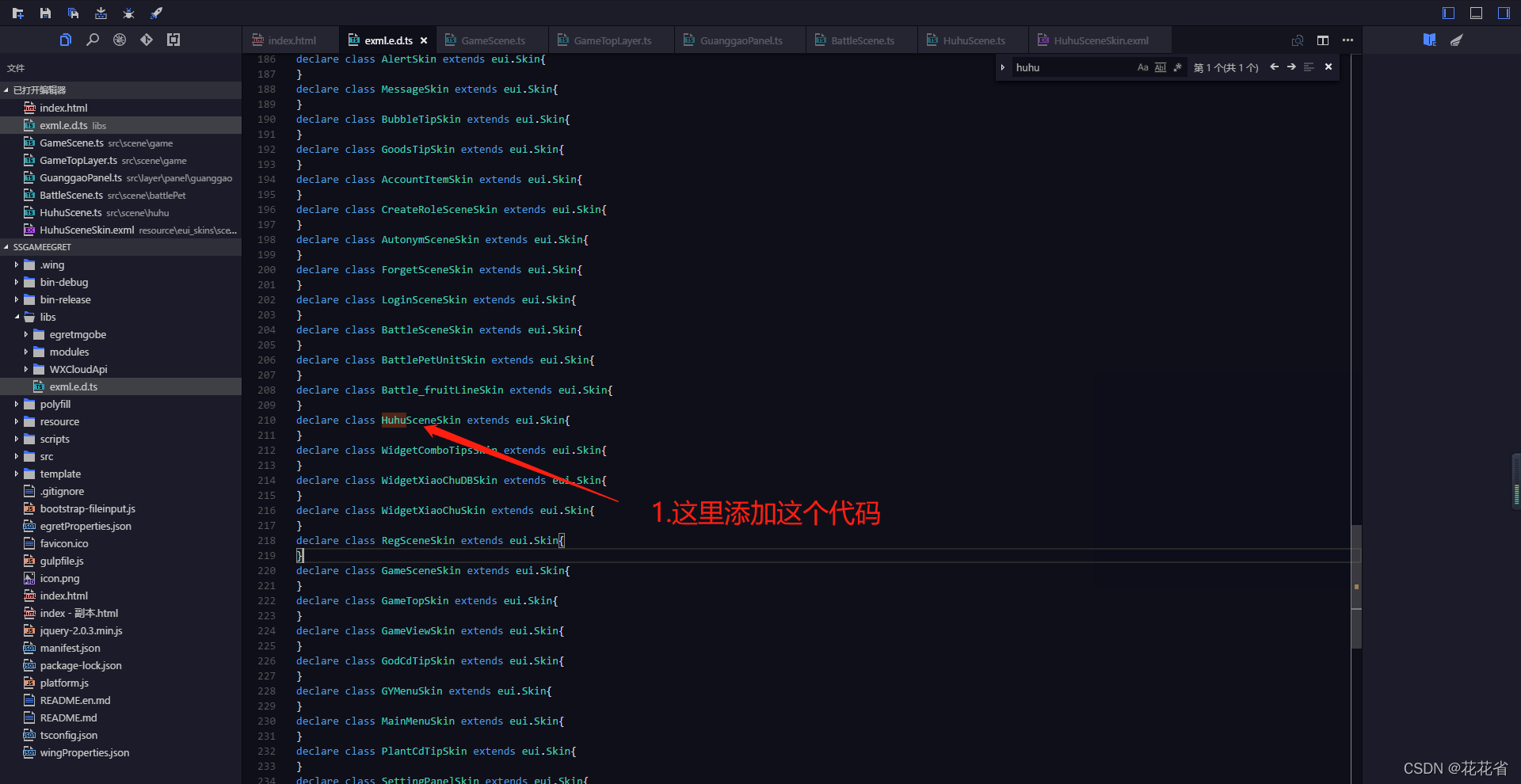
白鹭egret添加新页面教程,如何添加新页面
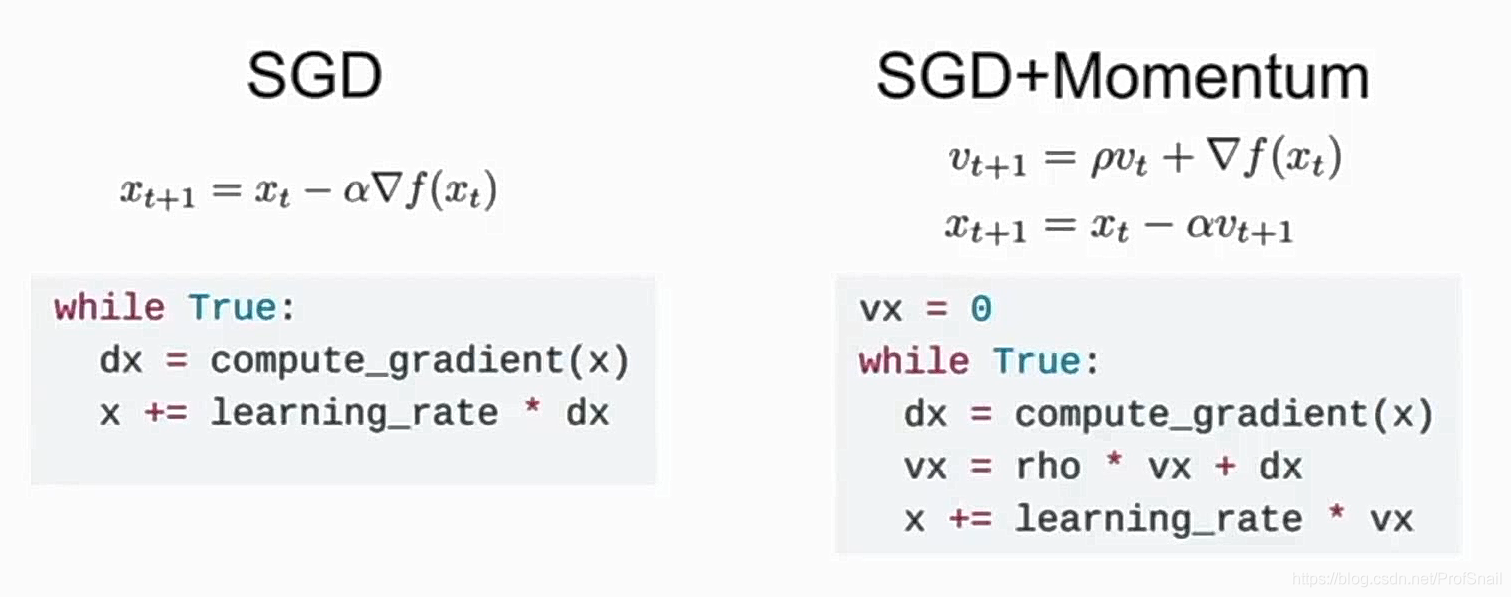
cs231n learning record

typescript63-索引签名类型
随机推荐
【FAQ】CCAPI兼容EOS相机列表(2022年8月 更新)
unity 将Text批量替换为TextMeshProUGUI
VS Code私有服务器部署(私有化)
日本卫生设备行业协会:日本温水喷淋马桶座出货量达1亿套
After docker is deployed, mysql cannot connect
在STM32中使用printf函数
MySQL: basic part
开启防火墙iptable规则后,系统网络变慢
export使用
技术分析模式(十)头肩图案
Technical Analysis Patterns (11) How to Trade Head and Shoulders Patterns
typescript68-索引查询类型(查询多个)
Redis
typescript66-分析partial的实现
【JVM调优】Xms和Xmx为什么要保持一致
边缘盒子+时序数据库,美的数字化平台 iBUILDING 背后的技术选型
八大排序之堆排序
超简单的白鹭egret项目添加图片详细教程
D45_Camera assembly Camera
技术分析模式(七)发挥差距