当前位置:网站首页>[Chongqing Guangdong education] National Open University spring 2019 1396 pharmaceutical administration and regulations (version) reference questions
[Chongqing Guangdong education] National Open University spring 2019 1396 pharmaceutical administration and regulations (version) reference questions
2022-07-04 16:07:00 【yuyueshool】
Test paper code :1396
Pharmaceutical administration and regulations ( Ben ) test questions
2019 year 7 month
One 、 Single topic selection (35 topic , Each question 2 branch , common 70 branch )
1. It has dual goals , Both cultivate pharmaceutical talents , The organization that also released pharmaceutical research results is ( ).
A. Pharmaceutical education organization B. Pharmaceutical research organization
C. Drug administration organization D. Pharmaceutical associations
2. In charge of the National Drug Administration , Development of drugs 、 production 、 circulation 、 The departments that implement unified supervision and management in the use and other links are ( ).
A. Health Administration B. The Administration Department of traditional Chinese Medicine
C. Business Administration Department D. The State Drug Administration
3. Drug manufacturers press ( ) It is divided into chemical and pharmaceutical factories ( Chemical APIs and their / Or the pharmaceutical factory ), Traditional Chinese medicine factory ( Traditional Chinese medicine decoction piece factory 、 Chinese patent medicine factory ), Biochemical pharmaceutical factories and biotech pharmaceutical companies that mainly produce genetic engineering products .
A. The forms of ownership of means of production are different B. The type of drugs produced
C. Measures for the administration of drug classification D. The size of the enterprise
4. Founded on 1907 year , One of the earliest established academic groups in China is ( ).
A. China traditional Chinese Medicine Association B. China prescription drug Association
C. Chinese Pharmacist Association D. Chinese Pharmaceutical Association
5. The valid period of licensed pharmacist registration is ( ).
A.1 year B.2 year
C.3 year D.5 year
6.《 Traditional Chinese Medicine 》 It is a general term for the medicine of all ethnic groups in China, including the medicine of Han and ethnic minorities , It reflects the Chinese nation's commitment to life 、 Knowledge of health and disease , A medical and pharmaceutical system with a long historical tradition and unique theories and technical methods , Refer to ( )
A. The doctor of traditional Chinese medicine B, traditional Chinese medicine
C. Chinese patent medicine D. Chinese medicine
7. The right preservation method for fresh medicinal materials is ( ).
A. cold storage 、 Sand storage 、 Tank storage 、 Biological preservation B. cold storage 、 Preservative 、 Tank storage 、 Biological preservation
C. cold storage 、 Sand storage 、 Preservative 、 Biological preservation D. cold storage 、 Preservative 、 preservatives 、 Biological preservation
8. The State Food and drug administration applies for the import of medicinal materials of endangered species or the first imported medicinal materials , Issued by ( ) Approval document .
A. One time B. Valid for half a year
C. Effective all year round D. Used multiple times
9. The following medicinal materials under first-class protection are ( ).
A. Velvet Antler ( Wapiti ) B. Velvet Antler ( Sika deer )
C. Bear bile D. Pangolin
10. Poppy shells should not be dispensed unilaterally , Each prescription should not exceed ( ).
A. Two day dosage B. Three day dosage
C. Five day dosage D. Seven day dosage
11. According to the content of drug information , Can be divided into ( ).
A. Text messages 、 Image information 、 Voice message
B. Multimedia information 、 Computer ( Electronics ) Information, etc
C. Pharmaceutical Science and technology information 、 Drug economic information 、 Information on drug policies and regulations 、 Drug use information
D. under advisement ( Before listing ) Drug information 、 Registered drug information and post marketing drug information
12. The following general names of drugs 、 The wrong description of the printing and marking of the trade name is ( ).
A. For horizontal labels , Must be marked in a prominent position within the upper third
B. For vertical labels , It must be marked in a prominent position within the left third of the range
C. Do not use cursive 、 Hard to recognize fonts such as seal script , Do not use italics 、 Hollow 、 Decorate the font in the form of shadow
D. The font color should be black or white , It forms a strong contrast with the corresponding light or dark background
13. Prescription drugs can be found in the State Council ( ) Medicine jointly designated by the drug administration department 、 Introduction to Pharmaceutical Journals , However, it is not allowed to publish advertisements in the mass media or advertise to the public in other ways .
A. Business sector B. Business department
C. Health Administration D. The state administration of radio
14. The following can be advertised ( ).
A. Narcotic drugs B. Preparations prepared by medical institutions
C. Special drugs for the army D. Medical apparatus and instruments
15. The following contents that can appear in drug advertisements are ( ).
A. National new drugs B. Safe, non-toxic and side effects
C. Insurance Company Insurance D. Drug approval number
16. The monitoring period of new drugs shall be calculated from the date of approval for production of new drugs , No longer than ( ).
A.5 year B.6 year
C.8 year D.10 year
17. The format of new drug certificate number is ( ).
A. Guoyao Zhun character H(Z、S、J)+4 Bit year +4 Bit sequence number
B.H(Z、S)+4 Bit year +4 Bit sequence number
C.H(Z、S) C+4 Bit year +4 Bit sequence number
D. Chinese medicine certificate H(Z、S)+4 Bit year +4 Bit sequence number
18. Adverse reactions not specified in the drug instructions refer to ( ).
A. Adverse drug reactions B. New adverse drug reactions
C. Serious adverse drug reactions D. Adverse drug group events
19. In the elements of drug production quality management system ( ) Including the corresponding organization 、 File system and sampling 、 Inspection, etc , Ensure necessary inspection of materials or products before release , Confirm that the quality meets the requirements . Choose ( ).
A. Quality objectives B. QA
C. The quality control D. Quality risk
20. The following are the process quality control methods ( ).
A. Cause and effect diagram B. Histogram
C. Correlation chart D. Permutation chart
21. The following belong to 《 Drug production license 》 The change of registration items is ( ).
A. Change of the person in charge of the enterprise B. Change of legal representative
C. Change of production scope D. Change of production address
22. Apply for commissioned production of drugs , from ( ) Apply .
A. The entrusting party is the State Drug Administration
B. The entrusted party shall report to the State Drug Administration
C. The entrusting party is the provincial drug supervision and administration department where it is located
D. The entrusted party shall report to the local provincial drug regulatory department
23. GMP Regulations ,A Suspended particles in class I clean area () The maximum allowable number is per cubic meter of air ( ).
A. 20 B.29
C.2900 D.3520
24. The on-site inspection adopts the team leader responsibility system , The inspection team is generally composed of no less than ( ) drug GMP Composition of inspectors , From medicine GMP Randomly select from the inspector Library , And should follow the principle of avoidance .
A.3 B.5
C.7 D.9
25. The main body of drug recall is ( ).
A. Drug regulatory department B. Drug manufacturers
C. Pharmaceutical trading enterprises D. Drug user
26. GSP Regulations , The records and vouchers of drug wholesale enterprises should be kept at least ( ).
A.1 year B.2 year
C.3 year D.5 year
27. GSP Regulations , Drugs that cannot be displayed in drug retail enterprises include ( ).
A. Class II psychotropic drugs 、 Toxic traditional Chinese medicine varieties and poppy shells
B. Narcotic drugs and psychotropic substances
C, Class II psychotropic drugs 、 Precursor chemicals and poppy shells
D. Psychotropic drugs and toxic drugs
28.《 Qualification certificate of Internet drug information service 》 The issuing department of is ( ).
A. The State Drug Administration B. Provincial Drug Supervision and administration department
C. Municipal Drug regulatory department D. County level drug supervision and administration department
29. The establishment of medical institutions must be obtained in accordance with the law ( ).
A.《 Medical institution license 》 B.《 Medical institution License 》
C.《 Medical institution License 》 D.《 Practice license of medical institution 》
30.( ) The above hospitals should establish a pharmaceutical management and pharmacotherapeutics committee .
A. Class A B. second level
C. Level three D. super
31. The shelf life of anesthetic prescriptions is ( ).
A.1 year B.3 year
C.3 God D.7 God
32. The following behaviors that do not belong to irrational drug use are ( ).
A. Repeat administration B. Combined medication
C. Inappropriate medication D. Insufficient medication
33. The limit of emergency prescription is generally ( ).
A. 15 Daily dosage B.7 Daily dosage
C.3 Daily dosage D.1 Daily dosage
34. basis 《 Regulations on the administration of pharmacy in medical institutions 》, Clinical pharmacists in tertiary hospitals shall not be less than ( ) name .
A.10 B.8
C.5 D.3
35.《 Prescription management method 》 Regulations , The general prescription is ( ) color .
A. Light red B. Light yellow
C. Light green D. white
Two 、 Multiple choice ( Missed selection 、 multi-select 、 No score for wrong choice .5 topic , Each question 4 branch , common 20 branch )
36. The technical support institution of drug supervision and management is an important part of drug supervision and management , Provide technical support and guarantee for drug administrative supervision . Specific include ( ).
A. China food and drug inspection and Research Institute 、 National Pharmacopoeia Committee
B. The drug evaluation center of the State Food and drug administration (CDE)、 State Food and Drug Administration food Drug examination and Inspection Center
C. Drug evaluation center of the State Food and drug administration ( National ADR Monitoring Center )、 In the state Drug variety protection Review Committee ( Health food evaluation center of the State Food and drug administration )
D. The administrative matters acceptance service and complaint reporting center of the State Food and drug administration 、 State Food and drug administration The Qualification Certification Center for licensed pharmacists of the State Administration of supervision
37. The conditions that researchers in charge of clinical trials should have include ( ).
A. Have corresponding professional and technical positions and medical qualifications in medical institutions , Have the required in the test plan Expertise and experience
B. Have rich experience in clinical trial methods or can get the academic guidance of experienced researchers in this unit
C. Be familiar with the data and literature related to clinical trials provided by the sponsor
D. Have the right to control the personnel participating in the test and use the equipment required for the test
38. The following descriptions about the purchase license of pharmaceutical precursor chemicals are incorrect ( ).
A. The State implements a purchase license system for pharmaceutical precursor chemicals
B. It can be used when purchasing pharmaceutical precursor chemicals 《 Proof of purchase 》 original script 、 Copy or fax
C.《 Proof of purchase 》 The scope of application is unlimited , Any organization can apply
D.《 Proof of purchase 》 It can only be used once within the validity period
39. according to 《 Announcement on several policies for the examination and approval of drug registration 》(2015 In the first 230 Number ) Regulations , You can queue separately , What accelerates the review and approval is ( ).
A. Prevention and treatment of AIDS 、 Malignant tumor 、 Application for registration of innovative drugs for major infectious diseases and rare diseases
B. Application for drug registration for children
C Application for registration of drugs for specific and multiple diseases of the elderly
D. Application for registration of innovative drugs transferred to China
40. The purchasing activities of drug wholesale enterprises should be “ Three certainty ” and “ An agreement ”, Include ( ).
A. Determination of the legal qualification of the supplier B. Determination of the legitimacy of purchased drugs
C. Determination of the legal qualification of the salesperson of the supplier D. Sign a quality assurance agreement with the supplier
3、 ... and 、 Matching questions ( Choose the best answer from the following options and fill in brackets .5 topic , Each question 2 branch , common 10 branch )
A- Prescription review ;B A patient ;C- The person in charge of the enterprise ;D- Head of the medical institution ;E- Licensed pharmacist .
41.GSP Regulations , The main person responsible for the quality of traditional Chinese medicine in drug wholesale enterprises is ( ).
42. GSP Regulations , Quality management positions and ( ) The responsibilities of the post shall not be performed by other post personnel
To perform on behalf of .
43. Enterprises that provide Internet drug trading services to individual consumers , Should have ( ) Responsible for online real-time consultation .
44. At this stage, the focus of pharmaceutical management in medical institutions in China is ( ) Centered .
45.( ) Serve as the pharmaceutical administration and pharmacotherapeutics committee ( Group ) chairman .
边栏推荐
- LeetCode 35. Search the insertion position - vector traversal (O (logn) and O (n) - binary search)
- Width accuracy
- 文本挖掘工具的介绍[通俗易懂]
- The new generation of domestic ORM framework sagacity sqltoy-5.1.25 release
- Understand the context in go language in an article
- Unity动画Animation Day05
- [learning notes] matroid
- Nine CIO trends and priorities in 2022
- Hexadecimal form
- Understand Alibaba cloud's secret weapon "dragon architecture" in the article "science popularization talent"
猜你喜欢

MySQL学习笔记——数据类型(2)
Redis' optimistic lock and pessimistic lock for solving transaction conflicts

这几年爆火的智能物联网(AIoT),到底前景如何?

压力、焦虑还是抑郁? 正确诊断再治疗
Detailed explanation of MySQL composite index (multi column index) use and optimization cases
PR FAQ: how to set PR vertical screen sequence?
Will the memory of ParticleSystem be affected by maxparticles
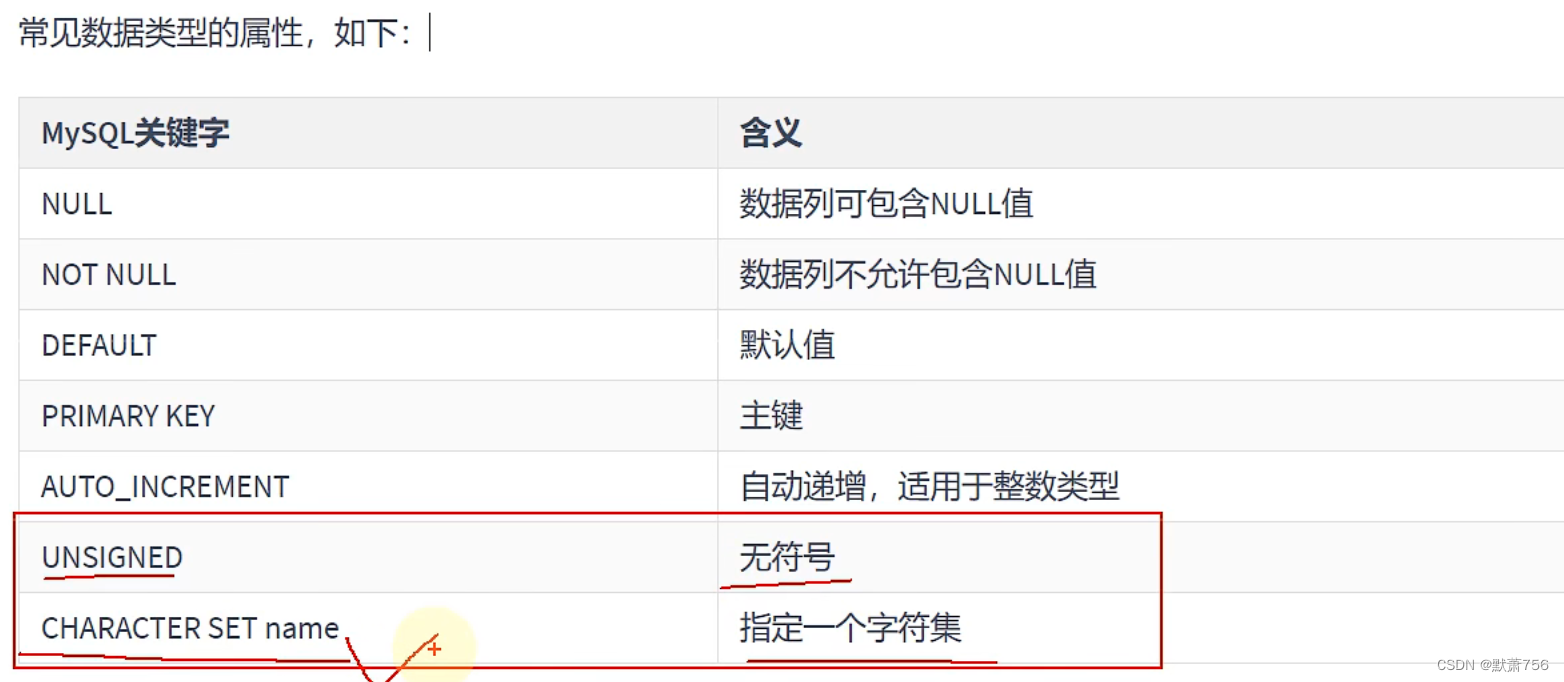
MySQL学习笔记——数据类型(数值类型)

165 webmaster online toolbox website source code / hare online tool system v2.2.7 Chinese version
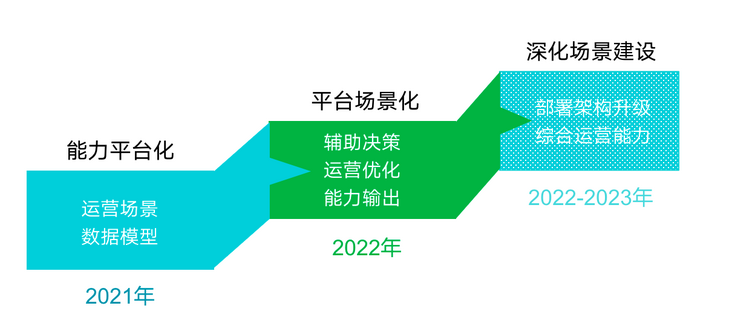
Case sharing | integrated construction of data operation and maintenance in the financial industry
随机推荐
Digital recognition system based on OpenCV
LeetCode 58. 最后一个单词的长度
Audio and video technology development weekly | 252
.Net 应用考虑x64生成
Summer Review, we must avoid stepping on these holes!
LeetCode 1184. 公交站间的距离 ---vector顺逆时针
The new generation of domestic ORM framework sagacity sqltoy-5.1.25 release
unity update 协程_Unity 协程的原理
Find numbers
The four most common errors when using pytorch
压力、焦虑还是抑郁? 正确诊断再治疗
直播预告 | PostgreSQL 内核解读系列第二讲:PostgreSQL 体系结构
Stress, anxiety or depression? Correct diagnosis and retreatment
How can floating point numbers be compared with 0?
Actual combat | use composite material 3 in application
【读书会第十三期】视频文件的编码格式
Usage of database functions "recommended collection"
Interpretation of the champion scheme of CVPR 2020 night target detection challenge
Ten clothing stores have nine losses. A little change will make you buy every day
Temperature control system based on max31865