当前位置:网站首页>Compliance and efficiency, accelerate the digital transformation of pharmaceutical enterprises, and create a new document resource center for pharmaceutical enterprises
Compliance and efficiency, accelerate the digital transformation of pharmaceutical enterprises, and create a new document resource center for pharmaceutical enterprises
2022-07-06 07:29:00 【Cloud box】
GMP/GSP It is the lowest threshold of the pharmaceutical industry , Although since 2019 year 12 month 1 The date of , From law to department regulation , No more GMP Relevant provisions of certification , But canceling certification is not the same as canceling GMP!
For non conformity GMP/GSP/GLP/GCP Required drug manufacturers , Will be ordered to rectify 、 Fines, etc , If the circumstances are serious, the drug approval certificate may even be revoked 、 Drug production license 、 Drug business license, etc .
Good and orderly documents are an essential part of the quality assurance system .

In the production and research and development of drugs , Lots of pictures of drugs 、 Clinical report 、 Scientific research materials and other documents are stored on the office computer or attached storage of each department , Not only is it difficult to summarize and control the data , There is also the risk of loss .
Pharmaceutical companies are facing increasingly cumbersome internal processes and increasingly stringent external regulatory compliance requirements , Cloud box replaces the traditional file management , Optimize the document business process of pharmaceutical enterprises through information means 、 Assist pharmaceutical enterprises to safely manage massive amounts of document data .

01、 Build a standardized enterprise document resource center
GMP The central guiding ideology of includes “ All work is documented ”, Make the document the of the enterprise “ law ”, Oppose plagiarism 、 Copy hard copy and execute documents, go through the motions or regard documents as decorations .
Cloud box helps pharmaceutical enterprises build a unified Document Resource Center , except GMP file , Also manage all kinds of administration 、 Research and development 、 Sales and other electronic documents , Such as management strategy 、 Drug formula 、 Sales data, etc , Form a unified enterprise document access portal . An update , Silent global synchronization .
Through the document resource platform GMP file management , Shorten the time of document effectiveness and distribution , Completely empty QA There are mountains of documents to be issued on the engineer's desktop 、 Records to be approved . Improve QA The efficiency of the Department .
02、 Leading safety leakage system
Huge R & D, production and sales data are the lifeblood of pharmaceutical enterprises .
Printed files 、 Uncontrolled electronic data is copied at will 、 Delete 、 Even unauthorized viewing , Even if the content is leaked, there is no trace , The security and integrity of pharmaceutical enterprise data cannot be guaranteed .
The document resource platform has a powerful and leading security system , Protect the security of pharmaceutical enterprise data , Include : The system is available online at any time 、12 Level authority control 、 Data handover of resignation documents 、 Document and personnel confidentiality 、 Dynamic watermark 、 Anti copy, anti copy, anti screenshot, etc , The authority is strict to control the upload of each document 、 visit 、 preview 、 download 、 Modify etc. .
03、 Quick intelligent retrieval of documents
Massive data can be searched to locate the target file , Document resource platform retrieval function , According to the file name 、 Quick retrieval of document content and number .
For example, by searching “SOP-QA”, Quickly find all quality assurance procedure documents .
There is no dead end in the query result 、 And not affected by equipment 、 Geographical constraints , Personnel can independently check the contents of documents at any time , Don't call QA Department inquiry .
04、 From drafting to approval , Online orderly circulation
from GMP Draft document preparation 、 QA to examine 、 Department review 、 The deputy general manager in charge will take effect after the final approval . The whole process can be carried out with the help of cloud box 【 workflow 】 Realize online automatic circulation approval .
One workflow can set multiple approval nodes , Approvers in the same approval node can approve documents at the same time 、 In the process of flow , Approvers support receiving reminders 、 Modify online 、 Add attachments 、 Electronic signature, etc .
After the process is over , Original document 、《GMP Document drafting / Revision notes 》、 The joint review opinions and other materials will be filed uniformly .
【 workflow 】 Show all unfinished 、 Pending approval , Let everyone involved in the process know the work progress in real time .
05、 Historical version management and review
Always display the latest version of the file in the document resource platform , This avoids QA Department members in the old version WORD Last revision .
Support at the same time WORD Modify online , Automatically generate a new version after saving and submitting .
The document resource platform version does not limit the number of versions , It supports commenting on each version , It is convenient to provide follow-up review clues , In order to investigate the history of suspected nonconforming products .
06、 Audit and log tracking
If in daily GMP Deviation found in the activity , You can return to the document resource platform system for audit tracking , View the operation log of key data , Check who and when , What operations have been performed on the data , And then help find the root cause of the deviation .
The log records of cloud box are all inclusive , As small as the login and logout of personnel , Management changes from administrators , comprehensive 、 Record every action on the system in an all-round way , Satisfy GMP Audit trail control requirements .
07、 Medical data integration and open platform
When the informatization construction of pharmaceutical enterprises reaches a certain degree , There are many internal information systems, which are easy to form data islands .
Cloud box is born with data integration genes , With platform level openness and connectivity , And OA、LIMS、ERP、MES And other information systems integration and docking , Unified identity authentication 、 Unified storage , Data De duplication .
The data of each system flows smoothly through the cloud box , To promote research and development 、 Production and operation management , Improve the management efficiency of all links , Realize the digitalization of business flow 、 automation .
Cloud box meets domestic and foreign GMP and GSP And other medical management norms , So far, it has served Yaojie Ankang 、 Ke Runxi medical 、 Kangqi medicine 、 Ruijing medical 、 Corechi Group 、ChemoPower And many other medical research enterprises , Committed to cutting-edge digital technology , Break the traditional document management in the pharmaceutical industry “ difficult ” and “ slow ” The problem of , Build an easy-to-use 、 Security 、 Intelligent quality document management system of pharmaceutical enterprises .
边栏推荐
- Résumé de la structure du modèle synthétisable
- LeetCode Algorithm 2181. Merge nodes between zero
- Detailed explanation | detailed explanation of internal mechanism of industrial robot
- word设置目录
- Get/post/put/patch/delete meaning
- On the world of NDK (2)
- Bit operation XOR
- Games101 Lesson 7 shading 1 Notes
- Methods for JS object to obtain attributes (. And [] methods)
- Bugku CTF daily question: do you want seeds? Blackmailed
猜你喜欢
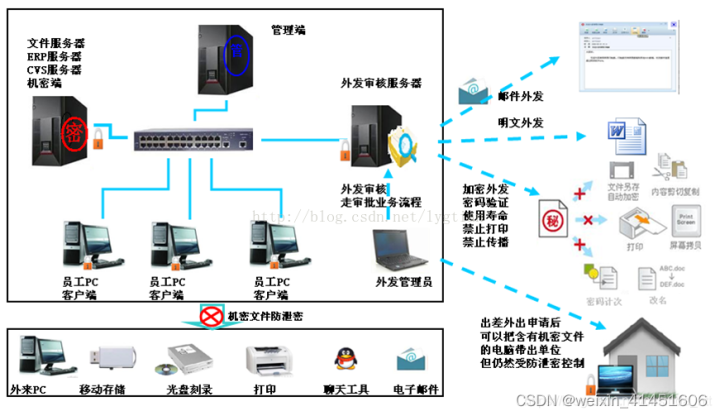
烧录场景下的源代码防泄密方案分享
Set picture annotation in markdown

Detailed explanation | detailed explanation of internal mechanism of industrial robot

The ECU of 21 Audi q5l 45tfsi brushes is upgraded to master special adjustment, and the horsepower is safely and stably increased to 305 horsepower

qt颜色与字符串、uint相互转换
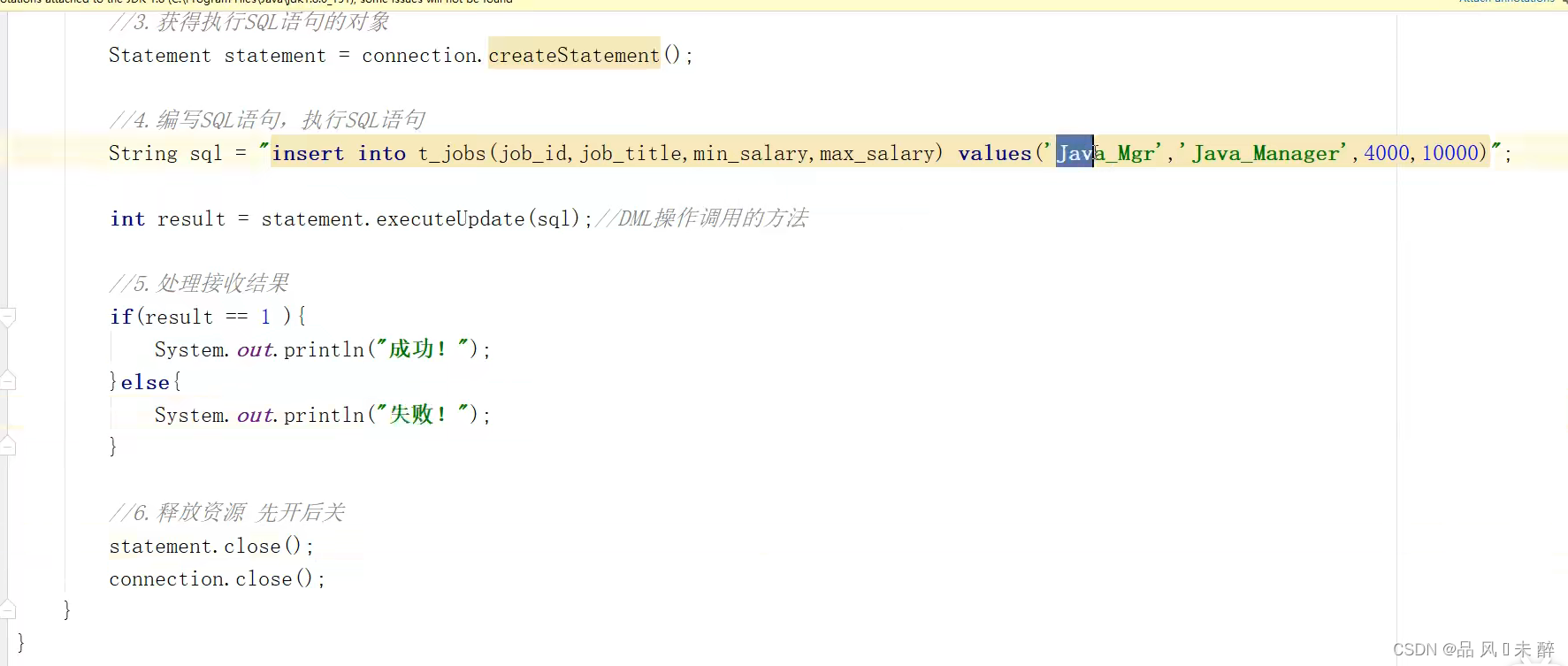
JDBC学习笔记
![When the Jericho development board is powered on, you can open the NRF app with your mobile phone [article]](/img/3e/3d5bff87995b4a9fac093a6d9f9473.png)
When the Jericho development board is powered on, you can open the NRF app with your mobile phone [article]
Relevant introduction of clip image

杰理之蓝牙设备想要发送数据给手机,需要手机先打开 notify 通道【篇】
Significance and measures of encryption protection for intelligent terminal equipment
随机推荐
GET/POST/PUT/PATCH/DELETE含义
C # create database connection object SQLite database
JMeter performance test steps practical tutorial
Summary of Digital IC design written examination questions (I)
Jerry's general penetration test - do data transmission with app Communication [article]
TypeScript 接口属性
Multi attribute object detection on rare aircraft data sets: experimental process using yolov5
Multithreading and concurrent programming (2)
Sharing of source code anti disclosure scheme under burning scenario
The differences and advantages and disadvantages between cookies, seeion and token
Typescript interface and the use of generics
Three no resumes in the software testing industry. What does the enterprise use to recruit you? Shichendahai's resume
word删除括号里内容
[window] when the Microsoft Store is deleted locally, how to reinstall it in three steps
QT color is converted to string and uint
杰理之BLE【篇】
The ECU of 21 Audi q5l 45tfsi brushes is upgraded to master special adjustment, and the horsepower is safely and stably increased to 305 horsepower
Uni app third party package configuration network request
Is the super browser a fingerprint browser? How to choose a good super browser?
When the Jericho development board is powered on, you can open the NRF app with your mobile phone [article]