当前位置:网站首页>In cooperation with the research team of the clinical trial center of the University of Hong Kong and Hong Kong Gangyi hospital, Kexing launched the clinical trial of Omicron specific inactivated vacc
In cooperation with the research team of the clinical trial center of the University of Hong Kong and Hong Kong Gangyi hospital, Kexing launched the clinical trial of Omicron specific inactivated vacc
2022-07-07 04:14:00 【sinat_ forty-one million six hundred and ninety-eight thousand 】
In recent days, , Hong Kong Gangyi Hospital ( abbreviation “Gleneagles” or “GHK”) Hold the launching ceremony of clinical trials , Kexing holding Biotechnology Co., Ltd (SINOVAC) Omicron specific inactivated novel coronavirus vaccine is used as a booster in healthy adults to enhance the safety and immunogenicity of vaccination , To provide scientific basis for formulating future vaccination strategies . This clinical trial was conducted by the clinical trial center of the University of Hong Kong (HKU-CTC) Led by the medical research team of , And cooperate with Gangyi Hospital .
This clinical trial plans to recruit 300 name 18 Healthy adult volunteers aged and over , Volunteers are required to have been vaccinated 2 Agent or 3 Agent inactivation or mRNA COVID-19 vaccine .
Assistant dean of the Li Ka Shing School of medicine, the University of Hong Kong ( recruit students )、 Clinical professor and director of infectious diseases department, Department of internal medicine, School of clinical medicine 、 Li Rujian, Professor of Huang Ruirong foundation ( Health Science pedagogy ) Professor Kong Fanyi said at the launching ceremony :“ as everyone knows , Vaccines are one of the most effective ways to deal with infectious diseases . at present , The variant of Omicron virus continues to ravage the world . It is hoped that this clinical trial can strengthen and effectively promote vaccine research and vaccination management .”
Dr. Zeng Qinglian said :“ As one of the research bases of the University of Hong Kong , We are very happy to participate in this clinical trial project , Contribute to the continuous research and development of vaccines and immunization strategies , Protect public health .”
Ms. luowenhui, director of business development of Kexing international, said :“ This time with Professor Kong 、 It is of great significance that the clinical trial center of HKU and Gangyi hospital cooperate to carry out the clinical trial of Omicron covid-19 inactivated vaccine enhancer . We hope to find solutions for Hong Kong and the world to fight against new virus variants through this test .”
Kexingyu 2021 year 12 Samples of Omicron mutants were obtained at the beginning of this month , Then actively promote novel coronavirus inactivated vaccine ( Omicron specific strain ) The development of . Preclinical studies based on animal models have shown , The vaccine is safe and effective . The clinical trial of strengthening immunity of Omicron strain was followed in 2022 year 4 month 12 Received the ethics review committee of the University of Hong Kong and Hong Kong Yi Hospital (IRB) approval , And in 4 month 14 It was approved by the Hong Kong pharmacy and poisons authority on the th , It is allowed to carry out enhanced immune clinical research on Omicron covid-19 in Hong Kong .
About Kexing holding Biotechnology Co., Ltd
Kexing biotechnology Holding Co., Ltd (SINOVAC) Is a leading biopharmaceutical company headquartered in China , Focus on the research of human infectious disease vaccine 、 innovation 、 Production and commercialization . The product portfolio of Kexing includes COVID-19 、H5N1 The flu ( Bird flu )、H1N1 The flu ( Swine flu ) And enteroviruses 71 (EV71) And other emerging infectious diseases , And for viral hepatitis 、 Seasonal flu 、 Pneumococcal pneumonia 、 Polio 、 Vaccines for other common infectious diseases such as chickenpox and mumps . The company is also constantly exploring opportunities in the international market , It is exporting vaccines to dozens of countries and international organizations .
About the clinical trial center of the University of Hong Kong
Clinical trial center of the University of Hong Kong (HKU-CTC) It is a one-stop clinical research management platform , It is committed to promoting the medical school of the University of Hong Kong to carry out clinical research projects in a professional manner . Since its establishment , The clinical trial center of the University of Hong Kong cooperates with global sponsors and research doctors , More than 1000 clinical studies initiated by the industry or researchers have been carried out .
About Hong Kong Gangyi Hospital
Hong Kong Gangyi hospital is a private hospital with multiple specialties , Located in Wong Chuk Hang, Southern District of Hong Kong Island , Equipped with 500 Beds , Provide advanced medical technology and comprehensive clinical services , Cover more than 35 There are specialties and branches . As the top private teaching hospital in Hong Kong , Gangyi hospital has made contributions to the training of medical professionals and the progress of clinical research . Gangyi hospital by IHH Medical group and Xinchuang Group Co., Ltd. jointly invest , The University of Hong Kong is the exclusive clinical partner .

[ Right up ] Assistant dean of the Li Ka Shing School of medicine, the University of Hong Kong ( recruit students )、 Clinical professor and director of infectious diseases department, Department of internal medicine, School of clinical medicine 、 Li Rujian, Professor of Huang Ruirong foundation ( Health Science pedagogy ) Professor Kong Fanyi ; Dr. Zeng Qinglian, CEO of Hong Kong joy Hospital ; Ms. Luo Wenhui, director of international business development of Kexing Asia Pacific ; Mr. You Guangzhi, managing director of the clinical trial center of the University of Hong Kong .( Photo : American business information )
边栏推荐
- termux设置电脑连接手机。(敲打命令贼快),手机termux端口8022
- 2022中青杯数学建模B题开放三孩背景下的生育政策研究思路
- Collection of idea gradle Lombok errors
- 使用切面实现记录操作日志
- Opencv third party Library
- Redis source code learning (30), dictionary learning, dict.h
- Force buckle ----- path sum III
- 【knife-4j 快速搭建swagger】
- Surpassing postman, the new generation of domestic debugging tool apifox is elegant enough to use
- Golang calculates constellations and signs based on birthdays
猜你喜欢
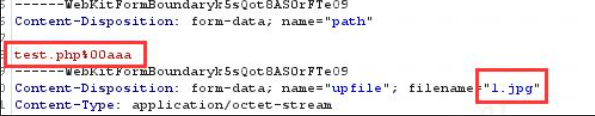
On file uploading of network security
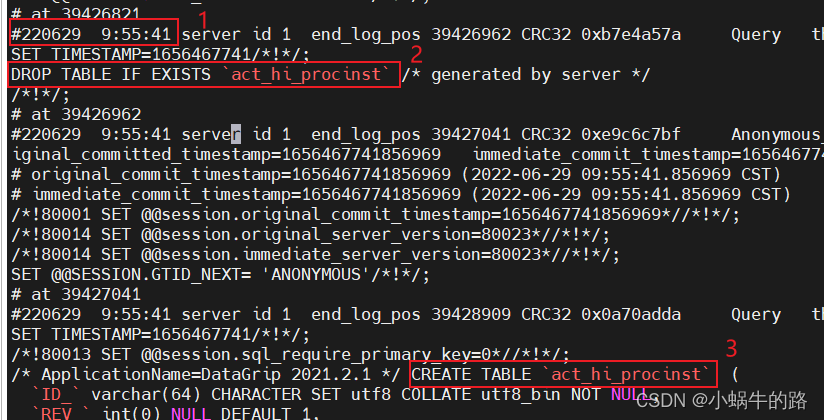
MySQL data loss, analyze binlog log file
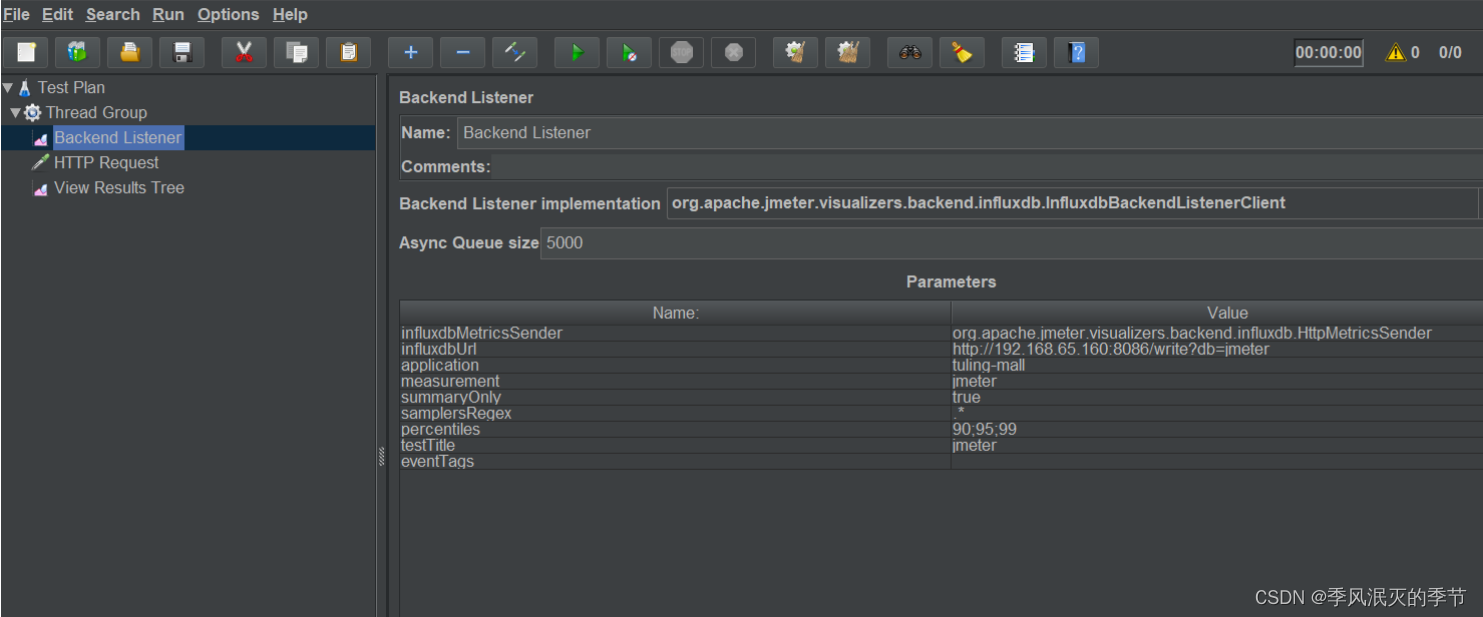
web服务性能监控方案
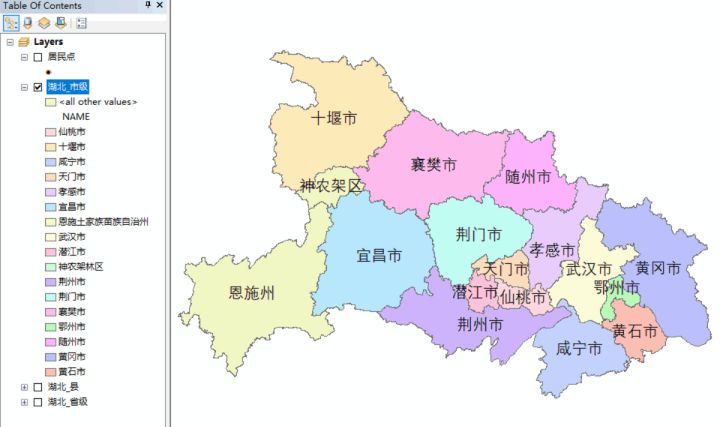
【ArcGIS教程】专题图制作-人口密度分布图——人口密度分析
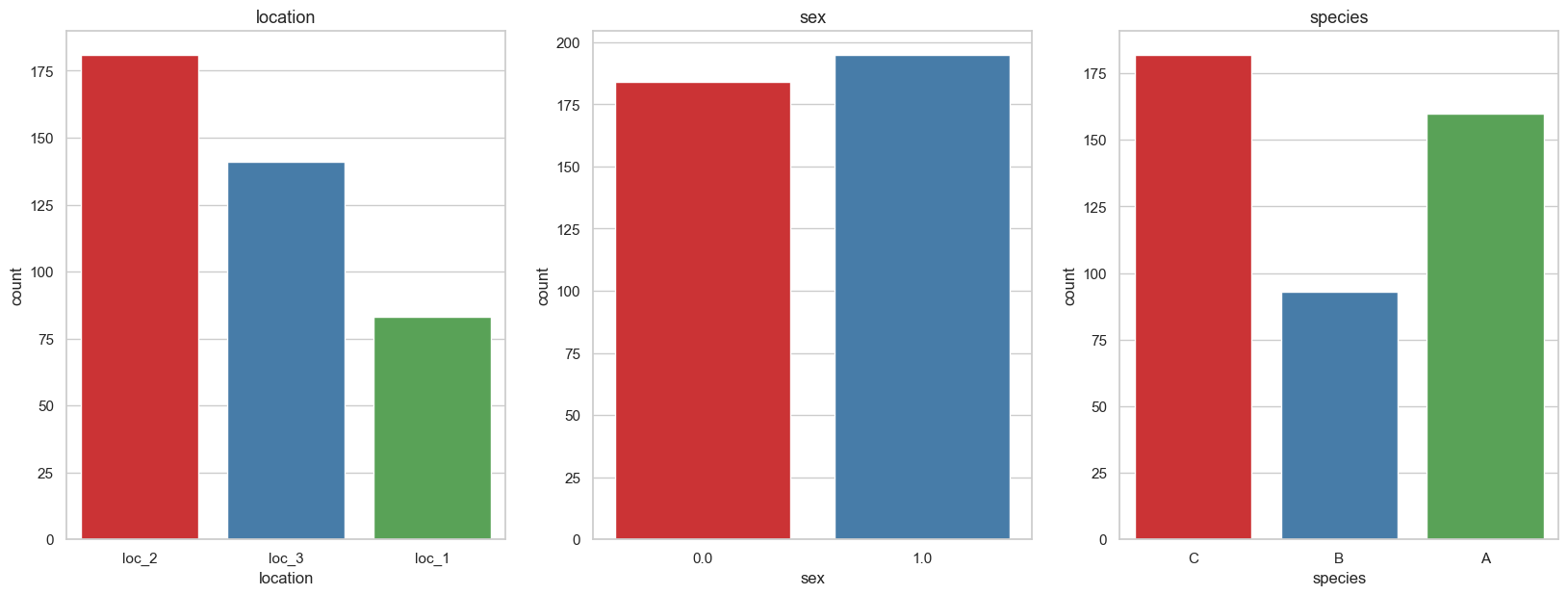
Machine learning notes - bird species classification using machine learning
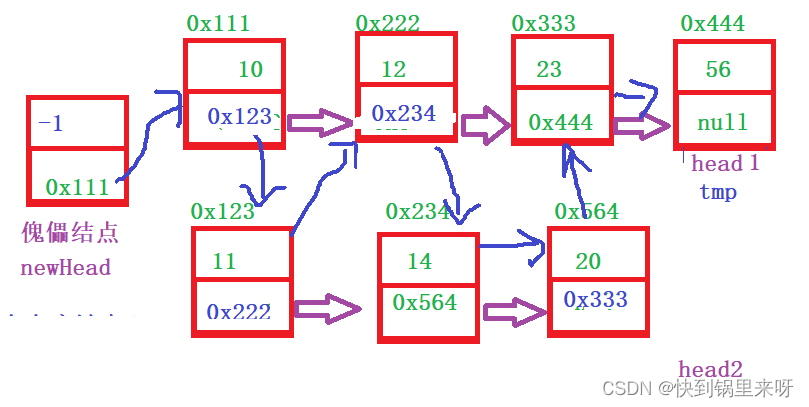
List interview common questions
【刷题记录】2. 两数相加
How to detect whether the MySQL code runs deadlock +binlog view
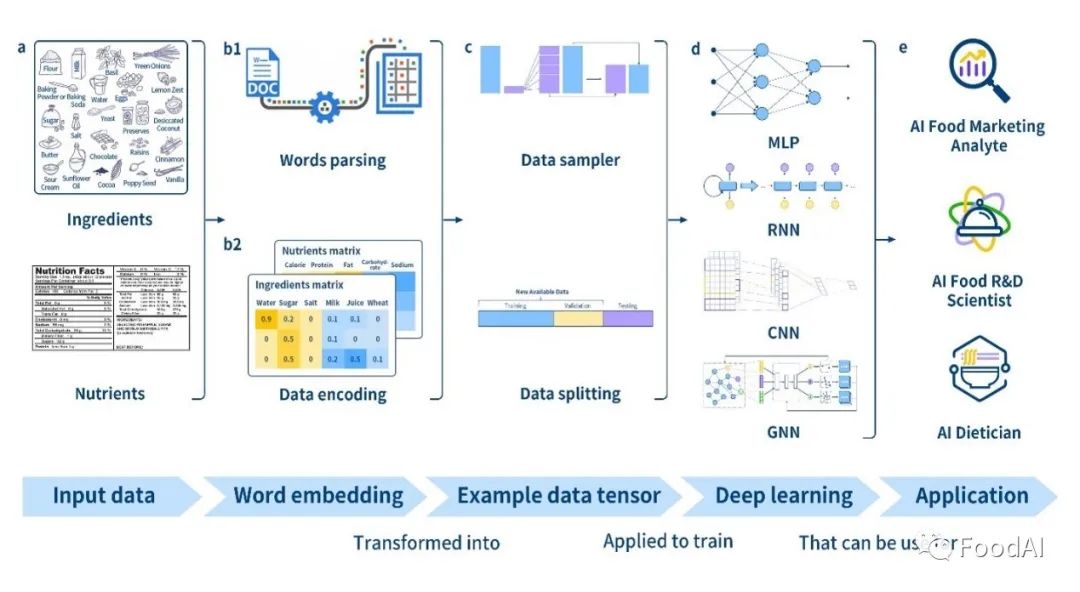
Food Chem | in depth learning accurately predicts food categories and nutritional components based on ingredient statements
用头像模仿天狗食月
随机推荐
一些常用软件相关
【刷题记录】2. 两数相加
How to write a resume that shines in front of another interviewer [easy to understand]
POJ training plan 2253_ Frogger (shortest /floyd)
Learn how to use js to merge two objects into one object assign()
Hangzhou Electric 3711 binary number
2022 electrician cup a question high proportion wind power system energy storage operation and configuration analysis ideas
Native MySQL
Class常量池与运行时常量池
Food Chem|深度学习根据成分声明准确预测食品类别和营养成分
接口自动化测试实践指导(中):接口测试场景有哪些
Enter the rough outline of the URL question (continuously updated)
Golang compresses and decompresses zip files
[leetcode]Spiral Matrix II
红米k40s root玩机笔记
高薪程序员&面试题精讲系列120之Redis集群原理你熟悉吗?如何保证Redis的高可用(上)?
机器人(自动化)课程的持续学习-2022-
2022中青杯数学建模B题开放三孩背景下的生育政策研究思路
使用 TiDB Lightning 恢复 GCS 上的备份数据
5年自动化测试,终于进字节跳动了,年薪30w其实也并非触不可及